When two or more drugs are combined into a single pill or formulation, it’s not enough to just match the brand name. You have to make sure the therapeutic equivalence is spot on-especially when doses differ between generics. This isn’t just about saving money. It’s about making sure a patient gets the same clinical result, every time, no matter which pharmacy they walk into.
What Therapeutic Equivalence Really Means
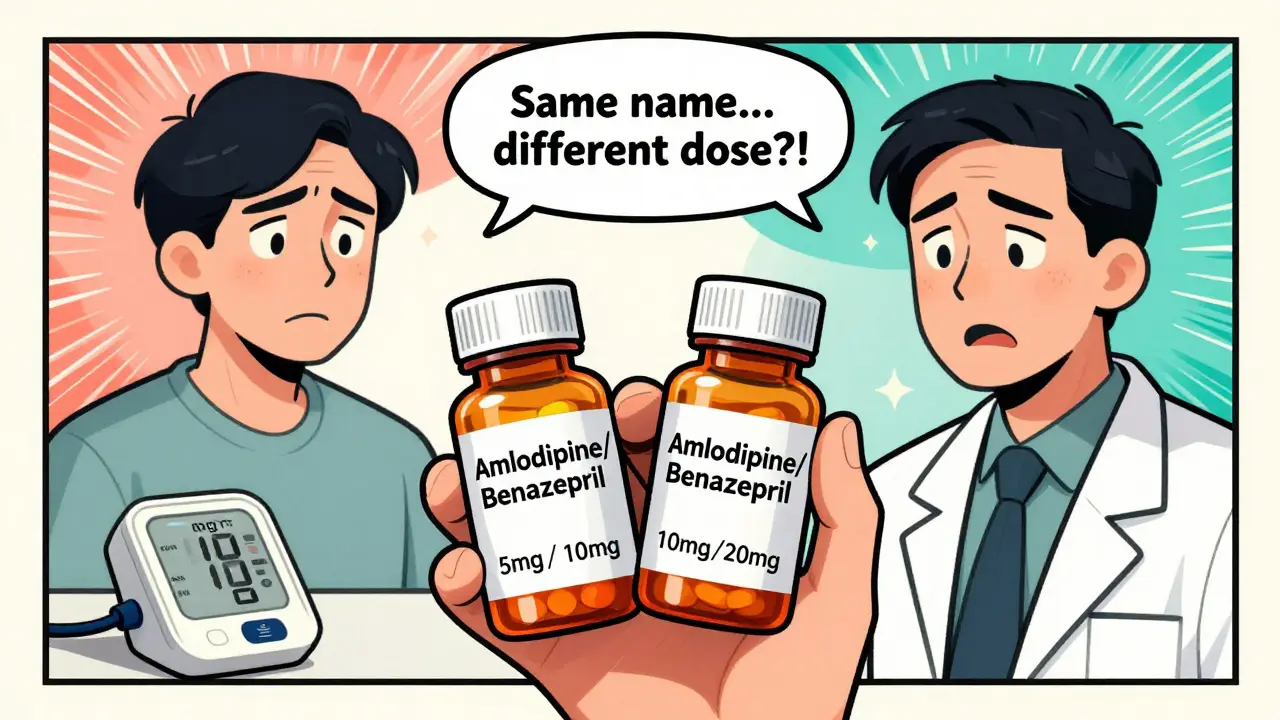
Therapeutic equivalence means two drug products-whether brand or generic-can be swapped without changing how well they work or how safe they are. The U.S. FDA tracks this in the Orange Book, a public database that rates thousands of drugs. If a product gets an ‘A’ rating, it’s considered interchangeable with the original. About 95% of FDA-approved generics fall into this category. But here’s the catch: this only applies when the active ingredients, strength, dosage form, and route of administration are identical. A 5mg/10mg combination of amlodipine and benazepril isn’t equivalent to a 10mg/20mg version, even if they’re made by the same company. Dose matters. And in combinations, even small differences can throw off the entire balance.Why Dose Equivalency Gets Complicated in Combinations
Imagine a patient taking tramadol and acetaminophen together for pain. The two drugs work in different ways-tramadol affects pain signals in the brain, while acetaminophen reduces inflammation. Together, they create a stronger effect than either alone. That’s synergy. But if you switch to a generic version with the same total dose, the ratio might be off. One generic might have 37.5mg tramadol with 325mg acetaminophen; another might use 50mg with 325mg. The total is close, but the balance isn’t. This isn’t just theoretical. A 2018 study in the Journal of Clinical Endocrinology & Metabolism found that 12% of patients switching between different levothyroxine generics-even though they were rated ‘A’-had abnormal thyroid levels. Levothyroxine has a narrow therapeutic index: too little doesn’t help; too much causes heart problems. When it’s combined with another drug, like a beta-blocker, even tiny absorption differences can become dangerous.The FDA’s TE Code System and What It Doesn’t Tell You
The FDA uses TE codes like ‘AB1’, ‘AB2’, or ‘B’ to show equivalence. ‘A’ means interchangeable. ‘B’ means there’s uncertainty-maybe the generic hasn’t been tested well enough, or it has a different inactive ingredient that affects how the drug is absorbed. But here’s where it gets messy: two products can both have an ‘A’ rating, yet still behave differently in combination. For example, rivaroxaban (a blood thinner) has seven generic versions, all rated ‘A’. But three use croscarmellose sodium as a filler, while four use sodium starch glycolate. These differences can change how quickly the drug enters the bloodstream. In a combination with aspirin or clopidogrel, that speed difference could mean the difference between preventing a clot or triggering a bleed. The FDA’s current system doesn’t account for these subtle formulation differences in combinations. It only checks the active ingredients. That’s a gap.
Real-World Mistakes Happen More Than You Think
A pharmacist in Ohio reported three dose-related errors in six months just from switching between different strengths of amlodipine/benazepril combinations. Patients were getting 5/20mg instead of 10/20mg-thinking they were equivalent because both were labeled “high blood pressure meds.” The result? Blood pressure spiked in two patients, one ended up in the ER. Another case from Allnurses.com involved a patient switched from Vytorin (ezetimibe/simvastatin) 10/20mg to a generic. The generic had the same total dose, but the absorption profile was different. The patient’s LDL cholesterol rose by 15%. The doctor didn’t catch it until the next check-up. The FDA’s own adverse event database recorded 247 incidents in 2022 tied to dose conversion errors in combination products. Nearly 40% involved heart medications. That’s not a glitch-it’s a systemic blind spot.How to Manage This Safely
There are three steps every clinician and pharmacist should follow:- Check the Orange Book for the TE code. Only use products with an ‘A’ rating.
- Match the exact dose combination. Don’t assume 5/10mg is interchangeable with 10/20mg. Look at the individual component strengths.
- Watch for NTI drugs. If the combination includes warfarin, levothyroxine, phenytoin, or cyclosporine, extra caution is needed. Use standardized conversion tables. Monitor blood levels if possible.
What’s Changing in 2026
The FDA is working on new tools. In early 2023, they released draft guidance for complex combination products-especially those where the dose-response isn’t linear. They’re also testing machine learning models that predict when a generic substitution might fail based on formulation details. Early tests are 89% accurate. Some experts are pushing for an ‘A*’ rating-reserved for combination products that’ve been tested across multiple strengths and formulations. Right now, only 3 out of 47 combination biologics have any equivalence framework at all. And in the future? Personalized medicine may change everything. By 2030, the NIH predicts that 30% of therapeutic equivalence decisions will include pharmacogenomic data-like whether a patient metabolizes drugs slowly or quickly. That means two people on the same generic combo might need different doses based on their DNA.Bottom Line: Don’t Assume Equivalence
Therapeutic equivalence is a powerful tool for cutting costs. But it’s not a free pass to swap anything with the same name. In combination products, the whole is more than the sum of its parts. Dose ratios, absorption rates, and inactive ingredients all matter. Just because two pills are rated ‘A’ doesn’t mean they’re interchangeable in every patient. Always verify the exact strength. Always check for narrow therapeutic index drugs. Always monitor patients after a switch. And when in doubt-stick with what’s working.What does an 'A' rating in the FDA Orange Book mean for combination drugs?
An 'A' rating means the generic combination product has been shown to be therapeutically equivalent to the brand-name version. This means it contains the same active ingredients in the same strengths, dosage form, and route of administration, and has passed bioequivalence testing. It can be substituted without expected differences in safety or effectiveness-unless it contains a narrow therapeutic index drug, where extra caution is needed.
Can two combination drugs with the same total dose be considered equivalent?
No. Total dose doesn’t guarantee equivalence. For example, a 5mg/10mg combination of amlodipine and benazepril is not interchangeable with a 10mg/20mg version, even if the total milligrams are doubled. The ratio between components matters. If the ratio changes, the clinical effect can change too-especially with drugs that work synergistically, like tramadol and acetaminophen.
Why are narrow therapeutic index (NTI) drugs riskier in combination products?
NTI drugs like warfarin, levothyroxine, and phenytoin have a very small window between an effective dose and a toxic one. Even minor differences in absorption between generics-caused by different fillers or coatings-can push levels into the dangerous range. In combinations, this risk multiplies because you’re now managing two drugs that may interact or affect each other’s metabolism.
Do generic combination drugs always cost less than brand-name versions?
Usually, yes-but not always. While most generic combinations cost 40-80% less than brand names, some complex formulations-especially those with multiple active ingredients or NTI components-may have higher manufacturing costs. In rare cases, the generic might be priced similarly to the brand due to limited competition or supply issues. Always compare actual pharmacy prices, not just assumptions.
How can pharmacists reduce errors when substituting combination products?
Use barcode scanning to verify exact strength and manufacturer. Maintain a list of high-risk combinations (NTI drugs, synergistic pairs). Train staff on the difference between total dose and component ratio. Implement a 72-hour follow-up protocol for patients switched to a new generic combination, especially if they have heart, kidney, or psychiatric conditions. Document every substitution clearly in the patient record.
Is therapeutic equivalence the same across all countries?
No. The FDA’s Orange Book system is specific to the U.S. The European Medicines Agency (EMA) requires additional in-vivo studies for fixed-dose combinations where components have different absorption rates. Canada, Australia, and the UK have their own guidelines. A drug rated 'A' in the U.S. may not be considered interchangeable elsewhere. Always check local regulatory standards when managing international prescriptions.


siva lingam on 24 January 2026, AT 12:25 PM
lol so the FDA has a whole book for this and still people die from mixups? what a joke.